Abstract

F. Malfona and I. Tanasi contributed equally.
M. Bonifacio and S. Chiaretti contributed equally.
This work was supported by AbbVie, trough supply of venetoclax and navitoclax provided within the therapeutic nominal program (Italian DM 7/9/2017).
Introduction. Venetoclax (Ven)-based regimens for the treatment of relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL) are increasingly used based on the preclinical efficacy, especially in subsets with a poor outcome likelihood, such as R/R T-ALL - for which the therapeutic armamentarium is scarce - and in B-cell precursor ALL (BCP-ALL) patients (pts) who relapse after first salvage treatment or after an allogeneic hematopoietic stem cell transplantation (HSCT), displaying complete response (CR) rates ≤20%. The combination with low-dose navitoclax (Navi) provides a synergistic effect in inhibiting BCL2 family proteins.
Methods. We performed a multicenter retrospective analysis to evaluate the impact of the BCL2 and BCLXL/BCL2 antagonists Ven and Navi alone or in combination (VN) in adult R/R ALL or lymphoblastic lymphoma (LL) pts. The study population included pts considered eligible for treatment and for whom clinicians had submitted application for compassionate use of the drugs; the concomitant administration of chemotherapy with Ven or VN was allowed, at the investigators’ discretion. Primary objectives were overall response rate (ORR) and complete remission (CR) at day 28 of treatment (end of cycle 1) and monitoring for inacceptable toxicities. Secondary objectives were 1) detection of measurable residual disease (MRD) by flow cytometry (FCM) and/or ASO qPCR in responding pts; 2) impact of different doses of study drugs; 3) feasibility of subsequent allogeneic transplant (allo-SCT) or CAR-T-cells once in CR. Adverse events (AE) were reported using the NCI-CTCAE version 5.0.
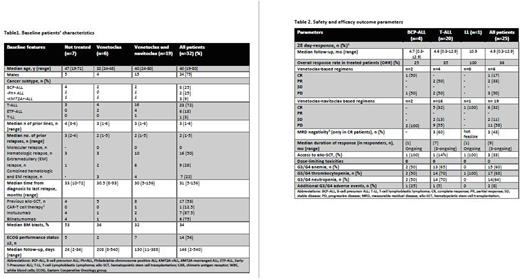
Results. We collected data of 32 pts affected by R/R ALL (n=31) or LL (n=1) from 17 Italian hematology centers. Median age was 40 years (range 19-80). Seventy-five % of pts were male. Eight pts (25%) had BCP-ALL, including 2 Philadelphia-positive ALL (Ph+ ALL) and 3 KMT2A-rearranged ALL (KMT2A-r ALL), 23 pts (72%) were affected by T-ALL of whom 6 had early T-cell precursor ALL (ETP-ALL). One patient had T-LL. At 1 June 2022, the median days of follow-up from treatment administration were 146 (2-540).
Pts had isolated hematologic (50%) or extramedullary (EM) disease (28%), or both (22%) (Table 1). Three pts (9%) were refractory to 3 prior lines of treatment. The median number of prior therapies was 3 (range 1-6). For BCP-ALL pts, 6 (75%) had received both inotuzumab and blinatumomab, and 1 also CAR-T-cells; in the whole cohort, 17 pts (53%) had already undergone an allo-SCT. Pts enrolled presented a median time from disease onset to treatment of 31 months (range 5-156), with a median of 2 previous relapses (range 1-5). Twenty-five of 32 pts received at least one of the study compounds (Table 2); 6 pts (19%) underwent Ven alone (n=2) or in combination with other agents (n=4) and 19 pts (59%) were treated with Ven in combination with Navi alone (n=9), or with additional chemotherapy schemes (n=10). The drug/s could not be administered in 7 pts because of fatal events prior to drug arrival.
Data at day 28 showed an ORR (CR+ PR) of 36%, higher in T-ALL pts (35%) than in BCP-ALL (25%). The combination of the two compounds appeared more effective, with a CR rate of 17% for Ven-based and 32% for VN-based treatments. Among CR pts, 43% achieved a MRD negativity. In the 9 pts who obtained a response, the duration was less than 3 months in 2, while it persisted at data cut-off in the remaining 7 cases; among responders, 33% underwent an allo-SCT. In 16 pts, there was no response (2 stable disease (SD), 14 PD), including 6 pts who underwent further chemotherapy. In the latter subgroup, only 2 pts were alive at data cut-off, 1 of whom in SD. Preliminary safety analysis showed that cytopenias (grade 3/4 thrombocytopenia (68%), neutropenia (64%) and anemia (60%)) were the main AEs, with no fatal AE.
Conclusions. Ven and Ven-Navi-based regimens appear tolerable and show preliminary efficacy in this heavily pre-treated and difficult-to-treat population of pts with ALL and LL, with 43% of pts in CR achieving a MRD negativity and 33% undergoing a subsequent allo-SCT. Survival sub-analyses are in progress and the study continues to accrue pts.
Disclosures
Papayannidis:Abbvie: Honoraria; Amgen: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Blueprint: Honoraria; Incyte: Honoraria; GlaxoSmithKline: Honoraria; Bristol Myers Squibb: Honoraria. Todisco:Abbvie: Consultancy, Other: Advisory Board; Janssen: Consultancy, Other: Advisory Board; Jazz Pharmacueticals: Consultancy, Other: Advisory Board. Del Principe:Gilead Sciences: Honoraria; Jansenn: Honoraria; Incyte: Honoraria. Chiaretti:Amgen: Other: advisory board; Incyte: Other: advisory board; Abbvie: Other: advisory board; Gilead: Other: advisory board; Pfizer: Other: advisory board.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal